ESTELITE BULK FILL FLOW
Innovative “edgeless” fillers ensure low-stress and safe curing
Article code: 12727
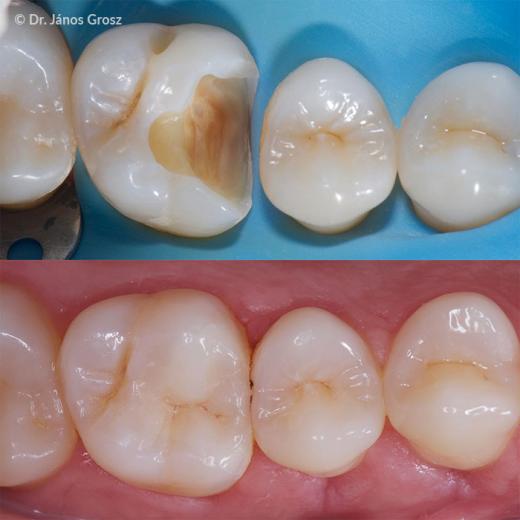
Case reports
ESTELITE BULK FILL FLOW in comparison
To activate the YouTube video, you must agree to the marketing cookies.
In comparison with common bulk fill materials ESTELITE BULK FILL FLOW shows superior handling properties. Thanks to the self-leveling effect of the composite and the shortened polymerisation time of just 10 seconds restorations are made quick and easy.
Awarded products - multiple certified service
- * Our Class IIa devices are certified as a medical device in the European Union under the Medical Device Directive 93/42/EEC by SGS CE1639, exclusively for the indication(s) detailed into the corresponding IFUs. Other non-medical uses ascribed to these devices are not within the scope of CE certification, and users should be aware product performance and/or safety has not been evaluated by SGS for those purposes.


 Change of refractive index during polymerisation with ESTELITE BULK FILL FLOW
Change of refractive index during polymerisation with ESTELITE BULK FILL FLOW