Composite Sub-micro-filler composites for perfect restorations
The development objective: Perfection in dental fillers
The extraordinary composites ESTELITE and OMNICHROMA are the result of an intensively sophisticated and innovative development concept. Size- and shape-controlled spherical sub-micron fillers, which are obtained in a fine manufacturing process, set new standards for aesthetic composite restorations. The long-standing development of the manufacturing process for fillers with special aesthetic, physical and user-friendly properties is the basis for the outstanding result.
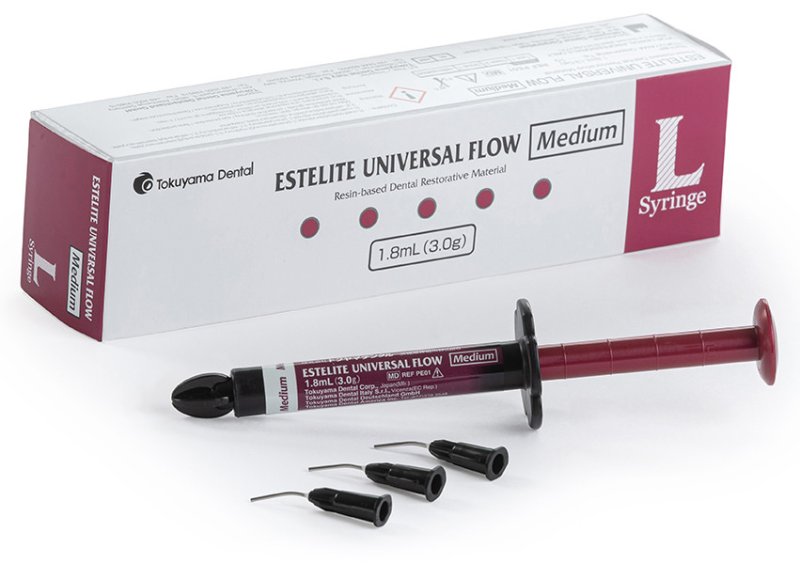
OMNICHROMA - a composite colour that adapts
Our dental composites have received numerous awards in the field of composite resin materials - including our universal composites OMNICHROMA and OMNICHROMA FLOW, with which we achieved a decisive innovation leap in composites fillings. With OMNICHROMA you only need one composite tooth shade for the entire VITA tooth shade range. After polymerisation, the OMNICHROMA filling material adapts continuously to the respective surrounding real tooth shade - all shades from A1 to D4 in just one syringe, in just one cap!
The benefits of composite tooth fillings from TOKUYAMA DENTAL:
✔ Unique chameleon effect due to sub-micron spherical fillers
✔ Perfect long-lasting aesthetics
✔ Optimal handling properties
✔ Outstanding physical properties
We would be pleased to advise you on the clinical application of our composite materials, from universal composites for the anterior and posterior region to flowable dental filling materials. Contact us now!